Production Method of Caustic Soda
Production Method of Caustic Soda In ancient Egypt, this material was produced by the reaction of sodium carbonate and hydrated lime according to the following reaction.
Na2CO3 + Ca(OH)2 → 2 NaOH + CaCO3
Over the years, other methods have replaced this old method, including Salvi’s method in 1861. But today, the best way to produce this material is to use the chloroalkali method industrially. In which sodium chloride solution is electrolyzed with the help of a diaphragm, membrane or mercury membrane to produce NaOH, hydrogen and chlorine.
2NaCl + 2H2O → Cl2 + H2 + 2NaOH
The membrane TB process is the most up-to-date and has economic and environmental advantages over the other two methods. However, in all three technologies chlorine is produced in the anode and hydrogen together with NaOH in the cathode (or in a separate reactor in mercury soln).
The main difference between these technologies is the difference in the method used to separate the anolyte and catholyte streams to prevent mixing. In the diaphragm method a separator and in the membrane method an ion exchange membrane performs this function. In the mercury method the cathode acts as a separator by forming an alloy of sodium and mercury, which then reacts with water in a separate reactor to form H2 and NaOH.
In the technologies mentioned, the final product is a mixture of sodium hydroxide and water, and additional steps must be taken to convert this product to a bump benefit.
We’ll get to know these three technologies first:
1- Production With Diaphragm Tuberculosis
This method was developed in the late 1800s. The diaphragm tubes have two anode and cathode segments separated by a permeable diaphragm (usually of asbestos fiber). Chlorine is formed at the anode and hydrogen and NaOH at the cathode. Sodium positive ions (cations) react with negative hydroxyl ions (anions) to produce NaOH.
The conversion rate of sodium chloride at each pass is approximately 50%. In the design of new asbestos tubes with polymeric materials, centuries have been replaced. Labor gas is also produced in a process similar to the mercury process. But with the exception that chlorine does not contain mercury from the chlorine condensate, other processes include chlorine cooling, drying, compression, and liquefaction.
Hydrogen gas can also be marketed or used as a fuel after going through a purifier or cooling process.
The NaOH solution has a concentration of between 10 and 12% sodium hydroxide and up to 18% sodium chloride. The NaOH solution is usually filtered to remove impurities and then reaches 50% concentration in a multifunctional evaporator. The resulting product will then be transferred to another unit to generate the jump profit.
Brine Purity
As mentioned earlier, brine is the starting material for the production of sodium (whether liquid or solid) by electrolysis of sodium chloride to produce NaOH. The purity of brine, or sodium chloride, is one of the important things to keep in mind. Brine purification is performed to obtain pure caustic soda and to prevent blockage and deposition in the diaphragm tubes.
The brine used usually contains impurities such as calcium, magnesium, and iron. When insoluble carbonates and hydroxides precipitate, these impurities are removed by adding lime and sodium carbonate.
Existing sulfate impurities are separated by BaCl2 or react with warm brine with OH- and CO3 -2 ions. After the brine has undergone certain processes to remove impurities, the impurities can be separated and then reacted with hydrochloric acid to neutralize.
Finally, saturated brine containing 324gr / lite of NaCl at 600 °C is fed to the diaphragm tubes for feed. The brine in the tubes electrolyzes the charge at a voltage of 3 to 4.5 volts.
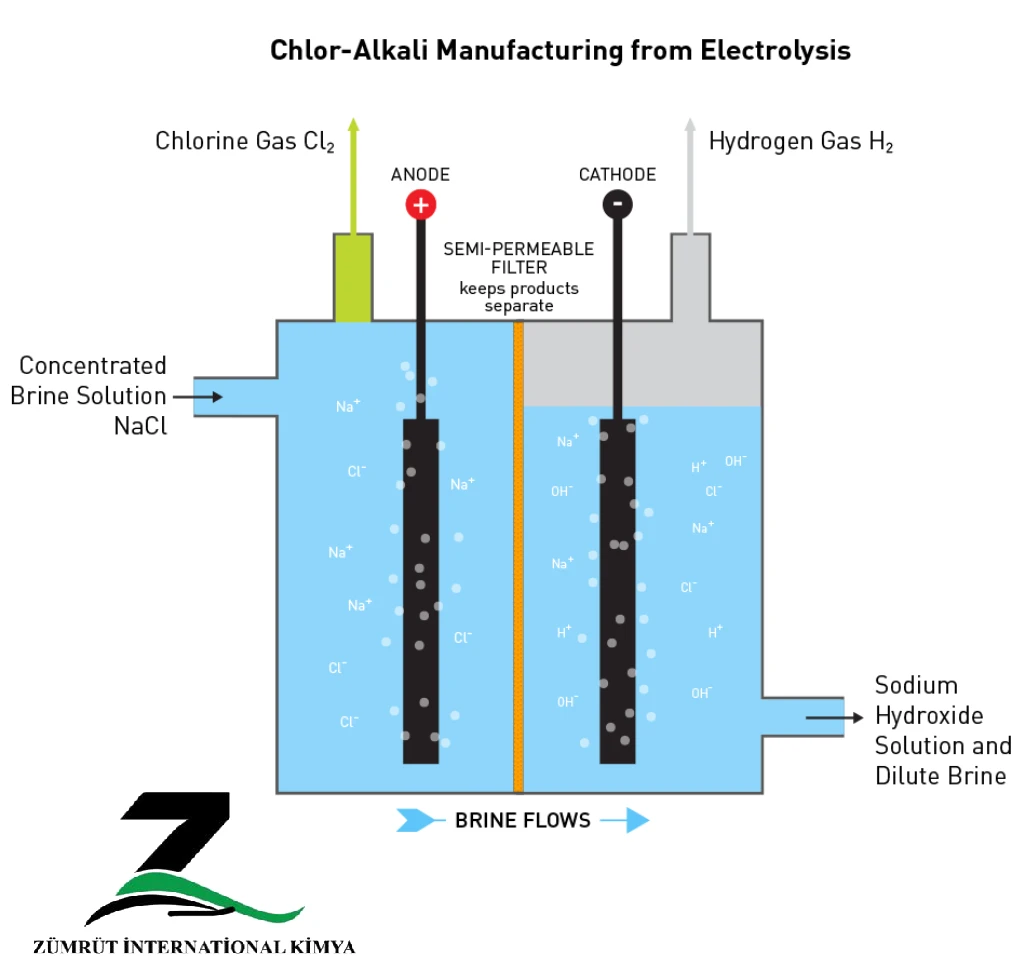
Description of The Electrolysis Process
Electrolysis at the anode is carried out at 0.07amp / cm2. The Na + ions formed at the anode move to the cathode and the H + and OH – ions are formed during the water reduction process. On the other hand, Cl-ions are directed to the anode, losing an electron, forming the chlorine molecule and being released as chlorine gas into the anode.
Since the discharge potential of chlorine ions is lower than that of OH- ions, Cl- ions are discharged at the anode and OH- ions remain in solution. Similarly, since the Na + discharge potential is greater than H+, H+ is discharged at the cathode and the Na + ion remains in solution.
It should be noted that chlorine can react with sodium hydroxide to produce sodium chloride and hypochlorite. Therefore, to prevent such a reaction, it is necessary that these two substances do not interact directly with each other.
Steam Control
The evaporative steam evaporates in a barometric condenser by contact with water or in non-contact surface condensers. Sodium chloride remains solid and is sent to the brine system. The salt separated from brine is returned to saturate the dilute soup (50% caustic solution contains 1% sodium chloride).
For specific applications such as silk production it is necessary to purify the obtained product. Low amounts of impurities can be eliminated by extraction or adsorption methods. For the production of 1000 kg of soda, 76% of the amount of raw material and energy required are as follows:
Evaporation & Salt Separation
After electrolysis, the caustic soda solution generally yields about 10-15% with some of the compound NaCl. The efficiency of salt degradation in cells is only 50% and half of the salt remains unreacted. This residual salt is recovered after condensation due to its low solubility in caustic soda.
The diluted soda caustic is concentrated to 50% in a 2 or 3-purpose evaporator, the salt is completely separated and then returned. The liquid from the separator is a 50% NaOH solution containing 2% NaCl and 0.1 to 0.5% dry salt.
The Final Evaporation
The 50% solution is concentrated in a large iron pot on direct fire. In this operation, approximately 99% of the available water is removed and the molten soda caustic forms at 5000 to 6000 ° C.
Another method is to dehydrate the precipitate of NaOH.H2O by adding ammonia which also helps to purify the caustic soda. If the 50% solution of aqueous ammonia is contacted in a pressure vessel in a non-equilibrium manner, the aqueous NaOH crystals will be separated from the ammonia solution.
The iron in the caustic soda is precipitated by sulfur and then the molten NaOH is pumped into the thin steel drums for centrifuge by pump.
By-products of the alkali chlorine process:
In addition to the production of NaOH, we also see the production of other important products, including hydrogen and chlorine.
1- Chlorine:
Chlorine is produced during a variety of processes including electrolysis, diako process, heating of uric acid and platonic chloride. All of these methods are expensive except for electrolysis, so chlorine is mainly produced by electrolysis.
Hot chlorine from the anode chamber contains a large amount of water vapor. As a result, to dry the existing water vapor, it is dried with the help of sulfuric acid scrubber. To dry the water vapors, a stone tower or steel tower with acid-resistant coating should be used. Finally, the dried chlorine is compressed under 30 to 80 psi with the help of one of the following temperature and pressure conditions.
High pressure (9 to 10 atm), cold water
Moderate (2 to 3 atm), cooling to -20 ° C
Low pressure (3 to 10 cm Hg), cooling to -40 ° C
For the production of liquid chlorine, rotary compressors containing sulfuric acid are used. The compression process is eliminated by water and finally the product is cooled to -29 ° C to produce liquid chlorine. This molding is done in two stages and in the second stage it is applied at a temperature of about -45 ° C. Finally, the liquid chlorine produced is transferred to the steel storage tank and then filled into stainless steel cylinders with a capacity of 50-100 kg for sale.
2. Hydrogen
Hydrogen obtained from the cathode is used as fuel in boilers or as a source of hydrogen.
2- Membrane cell process
The membrane cell method was rapidly expanded and adopted in the 1970s. These cells are considered to be the most efficient method for performing the chloroalkali process.
In a membrane TB, a perfluoropolymer membrane containing cation exchange groups separates the anode and cathode segments. This separator selectively transports sodium ions and prevents the transfer of hydroxyl ions from the catholyte portion to the andesite.
Saturated brine enters the anode and chlorine gas is released into the anode, with sodium ions migrating to the cathode with some water. Hydrogen is also ionized at the cathode part, producing a caustic soda by combining sodium and hydroxyl ions.
Due to the corrosive nature of chlorine gas, the anode must be made of a passive metal such as titanium, while the cathode can be made of steel. About 50% of sodium chloride is converted into cells. Finally, the residual brine is returned to the water purification system after chlorination.
The product of this process is also NaOH in combination with water, and additional steps must be taken to convert it to molten lead.
3 – Marcury Fried Cell
Like mercury diaphragm, the mercury tuberculosis method was developed in the late 1800s to generate perk profits. This method was widely used until mercury was discovered in the 1970s. The mercury cell has two parts: the analyzer and the electrolyzer.
The electrolyzer is a continuous steel rod with a gentle horizontal slope. The bottom of the cell is steel and the surrounding surfaces are made of rubber-coated steel, as well as the juice and mercury feed containers and the outlets are covered with a flexible rubber or rubber-coated steel.
Mercury flows through this cell and acts as a cathode, while Aben Mac flows over the mercury. The parallel plates of activated titanium anode are hung from the cell cover.
The flow through the cell breaks down the water and chlorine is formed at the anode and sodium metal at the cathode. Sodium is combined with mercury to form amalgam (an alloy of mercury and other material) and this amalgam flows from the electrolyzer to the catalyst.
Sodium-mercury amalgam is reacted with deionized water in a compound in the presence of a catalyst and hydrogen and NaOH are produced. Graphite is the most commonly used catalyst for this process. The catalyst is activated by iron, nickel or cobalt oxides or by molybdenum and tungsten carbides.
Much of the mercury is removed in a primary cooler that uses water as a coolant and returned to the electrolyzer.
Hydrogen Gas & Impurities
Hydrogen gas is cooled in a cooler to remove water vapor and mercury, to prepare hydrogen for sale or use as a cooling fuel to remove mercury.
The impurities in the solution can be removed by adding specific chemicals and then filtration. In most cases the produced caustic is sent to the storage unit or evaporated for greater concentration. In general, the caustic soda extracted from the catalyst has a concentration of 50% sodium hydroxide. As with previous processes, this output product also needs to be transferred to the relevant unit to generate the jump profit.
The released chlorine in the anode is cooled to remove water, sodium chloride and impurities. Usually the condensate produced at this stage is steam-stripped and returned to the brine system. After cooling, the chlorine gas is cooled further by sulfuric acid. Sulfuric acid can be used up to a concentration of 50-70% and dilute sulfuric acid can be recovered for sale, reuse or pH control.
Dry chlorine gas is compressed and converted to liquid. The liquefaction process gives rise to incompressible gases that are usually washed with soda or lime. This process produces a hypochlorite solution that is decomposed and can be sold or used in units. In these cells, about 12-16% of sodium chloride is converted.
Production of perk profits from liquid caustic soda
As noted above, the methods described in this section involved the production of NaOH solution. Perk is the solid form of this material, so additional processes to condense and produce solid rubber must be performed to convert the solution obtained from the previous three methods.
Liquid soda caustic (Lye) is sent to the Flaking unit for the production of perforated fluid using a fluid heater. Concentrated soda-free hot water (high temperature) in a rotary flicker is cooled by water and converted to flake caustic soda at melting temperature.
Production Capacity of Chlor-Alkali
Production by chlorine alkali in 2018 is estimated at about 9 million metric tons. Also this year, intercontinental caustic soda was 9.3 million DMT.
Applications of Sodium Hydroxide
This compound has many uses and is widely used in candle or soap making, paper production, detergents, and so on. It is a common product when producing chlorine in the alkali chlorine process. Eating it is dangerous and can damage human tissues. They use this material to eliminate fat and clogged pipes with fat. Solid or liquid NaCl can convert fats into soap and then dissolve them in water and clean surfaces of fats. When dissolving a drop of water in a jar, the reaction is very hot because the reaction is very exothermic.